PY-GCMS (Pyrolysis-Gas Chromatography-Mass Spectrometry): A Comprehensive Analysis of Principles, Advantages and Applications
Shanghai Jituo Material Technology Co., Ltd. provides PYGCMS testing services.
- PY-GCMS (Pyrolysis-Gas Chromatography-Mass Spectrometry) is a core technology for analyzing non-volatile, high-boiling-point, cross-linked, or macromolecular samples. It overcomes the limitation that traditional chromatography cannot directly analyze non-volatile samples. Through the combined logic of "pyrolysis conversion → chromatographic separation → mass spectrometry identification", it enables the complete interpretation of information from samples to their components.
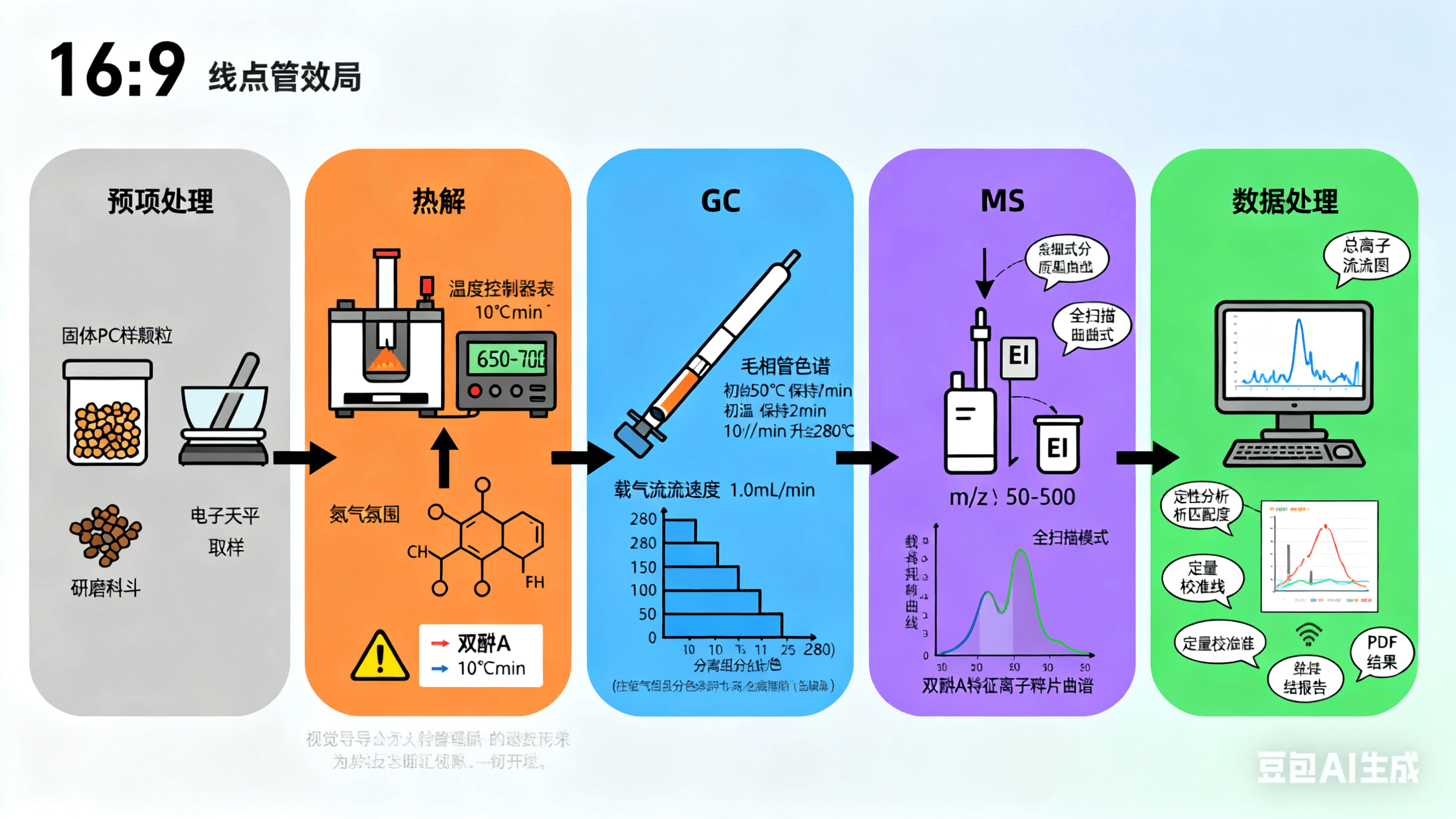
I. Core Principles of PY-GCMS Testing
The analytical process of PY-GCMS consists of three key steps—Pyrolysis, Gas Chromatographic Separation (GC), and Mass Spectrometric Detection (MS)—which are seamlessly connected via an interface to achieve the complete conversion from sample to component information. The specific principles are as follows: **1. Step 1: **Pyrolysis – Converting Non-Volatile Samples into Volatile Small Molecules Pyrolysis is the core pretreatment step of PY-GCMS. In essence, under strictly controlled temperature and atmosphere (usually an inert gas such as helium to prevent sample oxidation), high temperatures cause chemical bond cleavage in non-volatile macromolecular samples (e.g., polymers, resins, biological macromolecules), decomposing them into volatile small-molecule fragments (pyrolysis products). Key Control Parameters for Pyrolysis: Pyrolysis Temperature: Adjusted according to sample type (typically 300–1000°C). For example, 500–800°C for polymers and 400–600°C for biological samples. Insufficient temperature leads to incomplete sample decomposition, while excessively high temperature may cause secondary cracking (over-fragmentation of fragments, interfering with analysis). Heating Rate: Divided into "fast pyrolysis" (heating rate> 1000°C/s, e.g. laser pyrolysis) and "slow pyrolysis" (heating rate < 100°C/s, e.g., tube furnace pyrolysis). Fast pyrolysis reduces secondary reactions and more accurately reflects the original structure of the sample. Pyrolysis Atmosphere: Inert gases (helium, nitrogen) are commonly used to avoid sample oxidation. Reactive atmospheres (e.g., hydrogen, oxygen) may be used in specific scenarios for selective cleavage of specific chemical bonds. Example: Pyrolysis of Polyethylene (PE) When polyethylene (-(CH₂-CH₂-)ₙ) is pyrolyzed at approximately 600°C, C-C bonds break, generating a series of alkanes and alkenes with 4–18 carbon atoms (e.g., n-octane, 1-decene). These small molecules can then enter the subsequent gas chromatographic separation step.
2. Step 2: Gas Chromatographic Separation (GC) – Separating Pyrolysis Fragments by "Polarity/Boiling Point"
The mixture of small molecules produced by pyrolysis cannot be directly distinguished by mass spectrometry and must first be separated using gas chromatography (GC). Its principle relies on the difference in partition coefficients of different components in the sample between the "stationary phase" (coating inside the chromatographic column) and the "mobile phase" (inert carrier gas, e.g., helium) to achieve the separation of components one by one. Core Logic of Separation: Components with small partition coefficients (weak interaction with the stationary phase): Short retention time in the chromatographic column and elute first. Components with large partition coefficients (strong interaction with the stationary phase): Long retention time in the chromatographic column and elute later. Ultimately, the complex pyrolysis mixture is separated into a series of "single-component peaks" that enter the mass spectrometry detector in chronological order. Key Chromatographic Parameters: Chromatographic Column Type: Capillary columns are commonly used (e.g., DB-5MS, weakly polar, suitable for hydrocarbons and aromatics; DB-WAX, strongly polar, suitable for alcohols and esters). The selection depends on the polarity of the pyrolysis products. Column Temperature Program: Gradient heating (e.g., initial temperature 40°C held for 2 minutes, then increased to 300°C at 10°C/min) is used to balance the separation efficiency of low-boiling-point and high-boiling-point components.
3. Step 3: Mass Spectrometric Detection (MS) – Qualitative and Quantitative Analysis of Separated Components
After separation by GC, individual components enter the mass spectrometry detector (MS) with the carrier gas, and analysis is performed through the following processes: Ionization: Sample molecules are bombarded by high-energy electrons in an ion source (commonly an EI source, electron impact ionization), losing electrons to form molecular ions (M⁺). Meanwhile, molecular ions further break down into characteristic fragment ions. Mass Analysis: Fragment ions enter a mass analyzer (e.g., quadrupole, time-of-flight (TOF)), where they are separated based on differences in their mass-to-charge ratio (m/z). Signal Detection: The detector records the intensity of ions with different m/z values, generating a "mass spectrum" (x-axis: m/z, y-axis: ion intensity). Qualitative and Quantitative Analysis: Qualitative Analysis: The chemical structure of the component is determined by comparing the mass spectrum with a standard spectral library (e.g., NIST library). For example, a fragment ion with m/z = 57 corresponds to C₄H₉⁺ in alkanes, which can be inferred as a butyl structure. Quantitative Analysis: The content of the component is calculated by measuring the peak area of characteristic ions in the mass spectrum and using a calibration curve of standard samples.
4. Interface for Hyphenation: Enabling Seamless Connection of Pyrolysis-Chromatography-Mass Spectrometry
An interface is required to connect the pyrolyzer and gas chromatograph, with the core functions being: Maintaining a high temperature (usually higher than the boiling point of pyrolysis products) to prevent condensation of pyrolysis products at the interface. Controlling the carrier gas flow rate to ensure efficient entry of pyrolysis products into the chromatographic column. The commonly used interface type is a "direct transfer line" (made of quartz capillary with heating and temperature control), which has a simple structure and high transfer efficiency.
II. Core Advantages of PY-GCMS
Compared with single pyrolysis, GC, or MS technologies, PY-GCMS offers significant advantages in hyphenation: Applicable to Non-Volatile Samples: No complex pretreatment (e.g., derivatization) is required for samples. It can directly analyze non-volatile samples such as polymers, rubber, resins, coal, and biological tissues. High Resolution and Sensitivity: The efficient separation capability of GC combined with the high sensitivity of MS (detection limit down to the ng level) enables the differentiation of components with similar structures (e.g., phthalate plasticizers). High Qualitative Accuracy: By matching mass spectra with spectral libraries, the chemical structure of unknown components can be clearly identified, avoiding the error of "qualitative analysis based solely on retention time" in single chromatography. Comprehensive Information: A single analysis can simultaneously obtain information on the sample’s "pyrolysis behavior" (e.g., the relationship between pyrolysis temperature and product distribution), "component composition" (e.g., monomer units and additives of polymers), and "content information". III. Typical Application Fields of PY-GCMS The application scenarios of PY-GCMS focus on "component analysis, structural characterization, and source identification of non-volatile samples". The following are the core fields and examples:
1. Materials Science (Most Core Application)
(1) Polymer Analysis Polymer Type Identification: Pyrolysis products of different polymers have "fingerprint characteristics". For example: Pyrolysis of polypropylene (PP) mainly produces propylene monomers (m/z = 42) and dimers (m/z = 84). Pyrolysis of polystyrene (PS) mainly generates styrene monomers (m/z = 104). By analyzing the mass spectral characteristics of pyrolysis products, PP, PS, PE, and PVC (polyvinyl chloride, which contains HCl in pyrolysis, m/z = 36.5) can be quickly distinguished. Analysis of Polymer Additives: Detection of plasticizers (e.g., dibutyl phthalate (DBP), with characteristic pyrolysis ion m/z = 223), antioxidants (e.g., BHT (2,6-di-tert-butyl-p-cresol), m/z = 220), and flame retardants (e.g., decabromodiphenyl ether, m/z = 959) in plastics. Research on Polymer Degradation and Aging: By comparing changes in pyrolysis products between fresh samples and aged samples (e.g., UV aging, thermal aging), the degree of degradation is analyzed. For example, after PE aging, long-chain alkanes decrease while short-chain alkenes increase. (2) Analysis of Rubber, Resins, and Composite Materials Rubber Type Identification: Natural rubber (NR) pyrolyzes to produce isoprene (m/z = 68), while styrene-butadiene rubber (SBR) pyrolyzes to generate styrene (m/z = 104) and butadiene (m/z = 54). Resin Component Analysis: Pyrolysis products of epoxy resins and phenolic resins can reflect the type of curing agent (e.g., amine-based curing agents produce amine fragments via pyrolysis, while acid anhydride-based curing agents produce carboxylic acid fragments). Analysis of Matrix and Fillers in Composite Materials: For example, in carbon fiber composites, pyrolysis products of the resin matrix (e.g., epoxy resin) and carbon fiber (non-volatile, no pyrolysis peak) can be distinguished via spectra.
2. Environmental Science
(1) Analysis of Environmental Pollutants Microplastic Detection: Analysis of microplastics (e.g., PE, PS, PET) in water bodies, soil, and sediments. Qualitative analysis is conducted via characteristic peaks of pyrolysis products, and quantitative analysis is performed using the internal standard method (e.g., adding a known amount of acenaphthylene, m/z = 152, to correct recovery rate). Source Tracing of Persistent Organic Pollutants (POPs): For example, the pyrolysis characteristics of polychlorinated biphenyls (PCBs) and dioxins in soil can be used to trace pollution sources (e.g., industrial emissions, waste incineration). Analysis of Biomass Combustion Products: In aerosols generated by forest fires and straw combustion, guaiacol (m/z = 124) and syringol (m/z = 154) produced by lignin pyrolysis can be used as markers of combustion sources. (2) Analysis of Solid Waste Detection of organic pollutants (e.g., dioxins, polycyclic aromatic hydrocarbons (PAHs)) in fly ash from waste incineration. Identification of the composition of plastic waste to provide a basis for waste classification and recycling.
3. Food and Agricultural Products
(1) Analysis of Migrated Substances from Food Packaging Materials Detection of harmful substances migrating from food packaging (e.g., plastic films, paper packaging) into food, such as plasticizers (DBP, DEHP) in plastics and benzene compounds (benzene, toluene, with m/z = 78 and 92, respectively) in inks. (2) Analysis of Food Components and Quality Characterization of Macromolecular Components in Food: For example, pyrolysis products of starch (glucose derivatives, m/z = 126) can reflect the ratio of amylopectin to amylose. Identification of Food Fraud: For example, determining whether fructose-glucose syrup is added to honey (pyrolysis of fructose-glucose syrup produces furan compounds, m/z = 68, while pure honey mainly contains pyrolysis products of fructose and glucose). Analysis of Agricultural Product Processing Processes: For example, during coffee bean roasting, changes in pyrolysis products of cellulose and hemicellulose (e.g., furans, pyrazines, which affect coffee flavor) with roasting degree.
4. Archaeology and Cultural Heritage Conservation
(1) Identification of Cultural Heritage Materials Analysis of Lacquer Layers on Ancient Lacquerware: The main component of natural raw lacquer (urushiol) is a long-chain alkyl compound containing phenolic hydroxyl groups. Its pyrolysis produces phenol fragments (m/z = 94) and long-chain alkanes (m/z = 57, 71), which can be distinguished from modern synthetic lacquers (e.g., polyurethane lacquers). Identification of Textile Fibers in Ancient Relics: Cotton fibers (cellulose) produce glucose derivatives via pyrolysis, while wool fibers (protein) produce amino acid fragments (e.g., glycine, m/z = 75). Analysis of Binders in Ancient Pigments: For example, binders (animal glue, plant glue) in pigments of murals. Pyrolysis of animal glue (protein) produces nitrogen-containing fragments (m/z = 44, CO₂; m/z = 59, amides), while pyrolysis of plant glue (polysaccharide) produces furan compounds. (2) Research on Cultural Heritage Aging and Restoration Analysis of Aging Degree of Cultural Heritage: For example, if a large number of aldehyde and ketone fragments (m/z = 44, 58) appear in the pyrolysis products of cellulose in ancient paper or silk, it indicates severe cellulose degradation. Evaluation of Compatibility of Restoration Materials: For example, when restoring ancient lacquerware, the selected synthetic resin should match the pyrolysis behavior of the original lacquer layer to avoid accelerated aging in the future.
5. Other Fields
Coal and Petrochemical Industry: Analysis of coal pyrolysis products (e.g., methane, ethane, aromatics) to evaluate coal combustion efficiency and gasification potential; component analysis of petroleum asphalt (e.g., pyrolysis characteristics of saturates and aromatics). Biomedicine: Analysis of macromolecular components (e.g., collagen, keratin) in biological tissues (e.g., bones, hair) for forensic identification or paleontological research. Electronic Waste Analysis: Detection of components of plastics (e.g., FR-4 resin) and flame retardants (e.g., brominated flame retardants) in circuit boards to provide a basis for resource recovery of electronic waste. IV. Technical Limitations and Precautions of PY-GCMS Complexity of Pyrolysis Products: Secondary cracking (e.g., further cleavage of macromolecular fragments) may occur at high temperatures, weakening the correlation between products and the original sample structure. This interference can be reduced by controlling pyrolysis temperature and heating rate. Requirement for Sample Homogeneity: If the sample is heterogeneous (e.g., uneven distribution of fillers in composite materials), multiple samples should be analyzed to avoid result deviations. Limitations of Spectral Library Matching: Some new compounds (e.g., customized polymers) have no matching spectra in standard spectral libraries, so their structures need to be deduced based on fragment rules of known structures. Dependence of Quantitative Accuracy on Standard Samples: A calibration curve made with standard samples with structures similar to the target component is required; otherwise, quantitative errors may occur due to differences in response. In summary, as a "destructive but information-rich" hyphenated technology, PY-GCMS has become a core analytical tool in fields such as material characterization, environmental monitoring, and cultural heritage conservation due to its ability to directly analyze non-volatile samples. Its application scenarios continue to expand with technological advancements (e.g., high-resolution mass spectrometry (HRMS), in-situ pyrolysis technology).
